UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of Registrant as Specified in Its Charter)
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
(Address of principal executive offices) (Zip Code)
(Registrant’s telephone number, include area code)
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2 below):
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 2.05. | Costs associated with Exit or Disposal Activities |
On April 8, 2022, Frequency Therapeutics, Inc. (the “Company”) announced a reduction in force (the “Reduction”) of approximately 30% of its workforce. The purpose of the Reduction, which was approved by the Board of Directors (the “Board”) of the Company on April 5, 2022, is to better align the Company’s workforce with the needs of its business and focus more of its capital resources on its clinical program for its lead candidate for hearing restoration (FX-322); a second pre-clinical program for hearing restoration (FX-345); and a pre-clinical program for remyelination in Multiple Sclerosis. These changes will preserve capital, ensuring that the Company is appropriately resourced to advance its pipeline of potential first-in-class treatments through key development milestones. These milestones are the completion of the Phase 2b study of FX-322, a Phase 1b study of FX-345, and a Phase 1 study in the Multiple Sclerosis program.
A majority of the Reduction has already taken place, and the remainder will be completed by July 31, 2022, though all impacted employees have been notified. The total costs related to the Reduction are estimated to be approximately $1.2 million in future cash outlays primarily related to severance costs and related expenses.
| Item 5.02. | Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers. |
On April 5, 2022, the Board appointed Richard M. Mitrano, the Company’s Vice President of Finance and Operations, as the Company’s principal financial officer and principal accounting officer, succeeding Peter P. Pfreundschuh, the Company’s former Chief Financial Officer.
Richard M. Mitrano, 51, has served as the Company’s Vice President of Finance and Operations since July 2016. From 2012 to 2015, Mr. Mitrano served as the Director of Finance and Operations of Semprus, where he oversaw all accounting and finance operations and provided strategic direction and oversight. Prior to Semprus, Mr. Mitrano was a contract Accounting Manager for Predictive Biosciences, Inc. (“Predictive”), a diagnostics company, from 2010 to 2012. Prior to Predictive, from 2008 to 2010, Mr. Mitrano served as Corporate Controller of Pioneer Behavioral Health, a company providing behavioral health services. Mr. Mitrano holds a B.A. in Accounting from Bentley University.
| Item 7.01. | Regulation FD Disclosure. |
On April 8, 2022, the Company posted an updated corporate slide presentation in the “Investors & Media” portion of its website at www.frequencytx.com. A copy of the slide presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K (the “Current Report”).
The information in Item 7.01 of this Current Report, including Exhibit 99.1 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such filing. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
The following Exhibit 99.1 relates to Item 7.01, and shall be deemed to be furnished, and not filed:
| Exhibit No. |
Description | |
| 99.1 | Frequency Therapeutics, Inc. Corporate Slide Presentation as of April 8, 2022 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| FREQUENCY THERAPEUTICS, INC. | ||||||
| Date: April 8, 2022 | By: | /s/ David L. Lucchino | ||||
| Name: | David L. Lucchino | |||||
| Title: | President and Chief Executive Officer | |||||

Exhibit 99.1 Pioneering a New Category in Regenerative Medicine Frequency Therapeutics Corporate Presentation April 2022

Forward-Looking Statements and Other Disclaimers This presentation contains forward-looking statements within the meaning of the process of clinical drug development and regulatory approval; limited experience Private Securities Litigation Reform Act of 1995. All statements contained in this successfully obtaining marketing approval for and commercializing product presentation that do not relate to matters of historical fact should be considered candidates; the results of earlier clinical trials not being indicative of the results forward-looking statements, including without limitation statements regarding the from later clinical trials; differences between preliminary or interim data and final timing and design of Frequency Therapeutics’ (the “Company”) new Phase 2b data; adverse events or undesirable side effects; disruptions at the FDA and other trial of FX-322, including the type of SNHL that the enrolled patients will have and regulatory agencies; failure to identify additional product candidates; new or the ability of design features to reduce bias, the interpretation and implications of changed legislation; failure to maintain Fast Track designation for FX-322 and such designation failing to result in faster development or regulatory review or the results and learnings of other FX-322 clinical studies, the acceptance by the FDA of particular endpoints in the Company’s trials, the treatment potential of FX- approval; costly and damaging litigation, including related to product liability, 322, FX-345, and the novel approach for remyelination in multiple sclerosis, the intellectual property or brought by stockholders; dependence on Astellas Pharma timing and progress of the FX-345 and remyelination programs, the sufficiency of Inc. for the development and commercialization of FX-322 outside of the United the Company’s cash, cash equivalents and short-term investments, estimates of States; misconduct by employees or independent contractors; reliance on third the size of the hearing loss population and population at risk for hearing loss, parties, including to conduct clinical trials and manufacture product candidates; estimates of the size of the population with multiple sclerosis, estimates of the compliance with laws and regulations, including healthcare and environmental, commercial opportunity of FX-322 and the impact on existing treatment health, and safety laws and regulations; failure to obtain, maintain and enforce paradigms, the ability of our technology platform to provide patient benefit, and protection of patents and other intellectual property; security breaches or failure the potential application of the PCA platform to other diseases. to protect private personal information; attracting and retaining key personnel; and ability to manage growth. These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve These and other important factors discussed under the caption “Risk factors” in known and unknown risks, uncertainties and other important factors that may the Company’s Form 10-K filed with the Securities and Exchange Commission cause actual results, performance or achievements to be materially different from (SEC) March 15, 2022 and its other reports filed with the SEC could cause actual any future results, performance or achievements expressed or implied by the results to differ materially from those indicated by the forward-looking statements forward-looking statements, including, but not limited to, the following: the impact made in this presentation. Any such forward-looking statements represent management’s estimates as of the date of this presentation. While the Company of COVID-19 on the Company’s ongoing and planned clinical trials, research and development and manufacturing activities, the Company’s business and financial may elect to update such forward-looking statements at some point in the future, markets; the Company has incurred and will continue to incur significant losses it disclaims any obligation to do so, even if subsequent events cause its views to and is not and may never be profitable; need for additional funding to complete change. These forward-looking statements should not be relied upon as development and commercialization of any product candidate; the Company’s representing the Company’s views as of any date subsequent to the date of this dependence on the development of FX-322; the unproven approach of the PCA presentation. platform; the lengthy, expensive and uncertain © Frequency Therapeutics, Inc. | 2
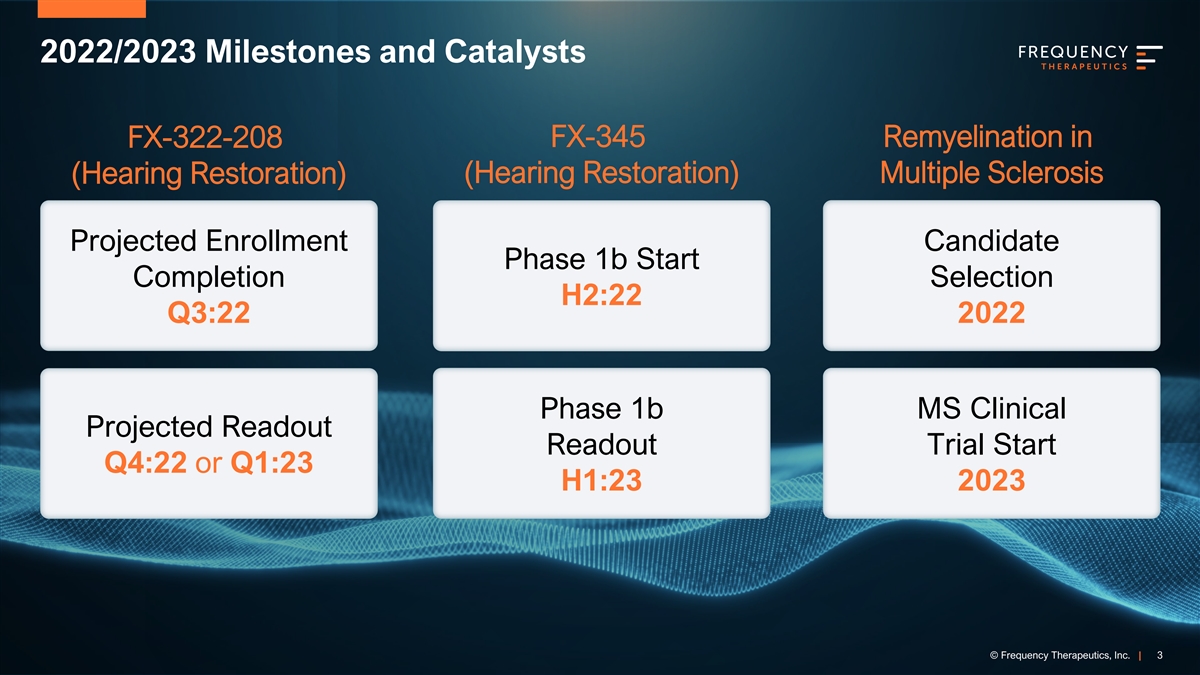
2022/2023 Milestones and Catalysts FX-345 Remyelination in FX-322-208 (Hearing Restoration) Multiple Sclerosis (Hearing Restoration) Projected Enrollment Candidate Phase 1b Start Completion Selection H2:22 Q3:22 2022 Phase 1b MS Clinical Projected Readout Readout Trial Start Q4:22 or Q1:23 H1:23 2023 © © F Fr req equ uen enc cy y T Th her era ap peu eut tiic cs s, I , In nc c. . | | 3 3

A Vision Built on Regeneration Since 2014, Frequency has been developing small molecule therapeutics to activate a person’s innate regenerative potential, within the body, to repair tissue and restore human function. © Frequency Therapeutics, Inc. | 4

Power of the Progenitor Cell Activation (PCA) Approach Ease of No Change to Harnessing Manufacturing Genome Innate Biology Use of small Activating native Progenitors molecules: no need programs, already located to remove or grow reducing safety within the cells ex vivo concerns target tissue © Frequency Therapeutics, Inc. | 5 © Frequency Therapeutics, Inc. | 5

A Series of Firsts in Hearing Restoration First PK/PD First clinical First speech First to show shown for a studies to show perception sustained hearing therapeutic hearing improvements improvements candidate improvements measured and continued improvements over time © © F Fr req equ uen enc cy y T Th her era ap peu eut tiic cs s, I , In nc c. . | | 6 6
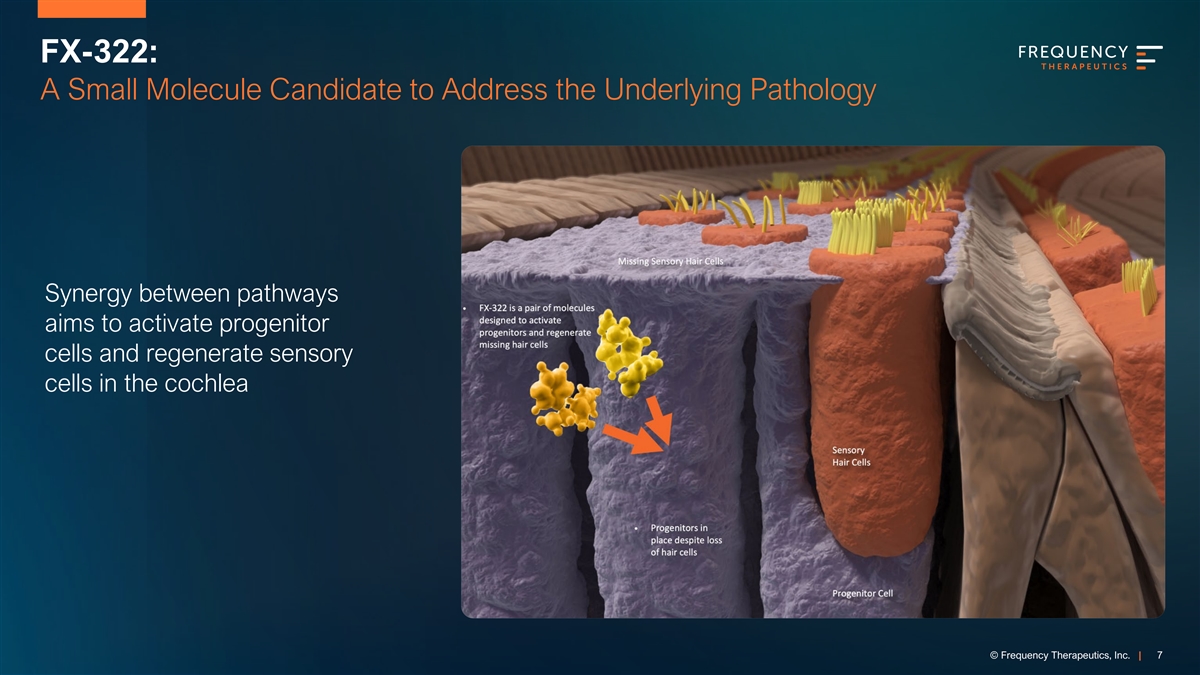
FX-322: A Small Molecule Candidate to Address the Underlying Pathology Synergy between pathways aims to activate progenitor cells and regenerate sensory cells in the cochlea © Frequency Therapeutics, Inc. | 7

FX-322: Directly Targeting the Regeneration of Sensory Hair Cells in the Cochlea The injection concentrates FX-322 FX-322 is administered via a standard in the cochlear region critical for intratympanic injection, a routine speech intelligibility procedure performed by ENTs © Frequency Therapeutics, Inc. | 8

Five FX-322 Completed Studies: 193 Treated Subjects Favorable Safety Profile with No Treatment-Related SAEs 2018 2019 2020 2021 2022 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 SINGLE-DOSE STUDIES SSNHL and NIHL Study (201) Phase 1/2 Durability Mild-Moderately Severe [18-65 y/o] Study (201-2) 1 201 Study of Administration Conditions (111) All Etiologies, Mild-Severe [18-65 y/o] 2 111 Age-Related Hearing Loss Study (112) Mild-Moderately Severe [66-85 y/o] 3 112 Severe Hearing Loss Study (113) All Etiologies [18-65 y/o] 4 113 MULTIPLE-INJECTION STUDY SSNHL and NIHL Study (202) [Mild-Moderately Severe [18-65 y/o] 5 202 = Data Readout © Frequency Therapeutics, Inc. | 9

FX-322-201, FX-322-111, FX-322-113 Single-Dose Safety Studies with Hearing Improvement Signal 2018 2019 2020 2021 2022 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 SINGLE-DOSE STUDIES SSNHL and NIHL Study (201) Phase 1/2 Durability Mild-Moderately Severe [18-65 y/o] Study (201-2) 1 201 Study of Administration Conditions (111) All Etiologies, Mild-Severe [18-65 y/o] 2 111 Age-Related Hearing Loss Study (112) Mild-Moderately Severe [66-85 y/o] 3 112 Severe Hearing Loss Study (113) All Etiologies [18-65 y/o] 4 113 MULTIPLE-INJECTION STUDY SSNHL and NIHL Study (202) [Mild-Moderately Severe [18-65 y/o] 5 202 = Data Readout © Frequency Therapeutics, Inc. | 10

Data from Controlled Studies (FX-322-201, FX-322-111) Improvement Shown in Speech Perception in Quiet with Single Dose Day-90 Word Recognition Scores Across Studies Phase 1/2 Study Phase 1b Study FX-322-111 FX-322-201 Overview Overview FX-322-201 Phase 1/2 FX-322-111 Phase 1b • Placebo-controlled, multi-center, • Compared different FX-322 40% randomized study administration conditions • Mild to moderately severe subjects, • Open-label, multi-center, age 18-65 (n=23) randomized study • NIHL/SSNHL • Mild to severe subjects, age 30% 18-65 (n=33) Study Results Study Results 20% • 33% of subjects achieved 10% or • 34% of subjects achieved 10% or greater absolute improvement in greater absolute improvement in word word recognition in treated ear recognition (WR) in treated ear 10% • Statistically significant and clinically • Statistically significant and clinically meaningful improvements in WR meaningful improvements in WR • No meaningful changes in • Favorable safety profile placebo group 0% • Favorable safety profile Placebo FX-322 Untreated FX-322 *Total of 33 patients enrolled in study, 32 subjects * * completed 90-day clinical assessment period (n=8) (n=15) (n=32) (n=32) © Frequency Therapeutics, Inc. | 11 % Exceeding Absolute 10%

FX-322 Phase 1/2 Durability Data: Patients Show Sustained Hearing Improvements 13-21 Months After Initial Dosing 50 50W Month 16** Month 21** Month 19** Month 13** Month 21** 47 Key Findings 50W 40 Preliminary evidence *25W 50W 39 indicating a durable benefit 38 38 50W 50W of hearing clarity 35 34 *25W 50W 30 30 29 50W Baseline - Correct words out of 50 26 50W Day 90 - Correct words out of 50 50W 22 20 20 1-2 Years - Correct words out of 50 50W 50W 16 50W 14 Three patients who had durable 12 10 improvements in intelligibility also had 50W pure tone audiometry improvements 7 of 10 – 15 dB at the highest frequency tested (8k Hz) 0 Subject 1 Subject 2 Subject 3 Subject 4 Subject 5 * 25W = 25 Word test performed outside an official study site at 13-18 months after dosing; results scaled to 50 words 50W = 50 Word test performed under a formal protocol at original study site at 18-21 months after dosing **Since FX-322 dosing © Frequency Therapeutics, Inc. | 12

Subjects in FX-322-111 Study Show Additional Hearing Improvements at Later Time Points Conducted longer-term, follow-up of FX-322-111 study subjects • 25 of 33 study subjects evaluated at 8-12 months following FX-322 dosing Results show some FX-322 dosed subjects accumulated hearing benefits over time • 3 subjects that had shown improvement trends in word recognition scores at day 90, achieved statistically significant scores when tested at the later time points To date, 8 of 32 evaluated study subjects have shown statistically significant improvements in speech perception scores in treated ears between 90 days and 1 year • No change observed in untreated ears © Frequency Therapeutics, Inc. | 13

Pooled FX-322 Data Shows Patterns of Response Single-dose Studies (201, 111, 112) Exceed 95% Confidence Interval % Exceeding Single-dose Studies Single-dose Studies 95% Confidence Interval (201, 111, 112) (201, 111 & 112) 25% 20 Exceeds 95% Confidence Interval 20% 10 14.1% 15% +10% 10% (5 words) 0 -10% (5 words) 5% 2.5% 2.1% 0% -10 Untreated Literature* Treated Treated Untreated Placebo and Placebo n = 71 n = 84 n = 13 n = 71 n = 97 95% confidence intervals established by Thornton & Raffin (1978) and modified by Carney & Schlauch (2007) © Frequency Therapeutics, Inc. | 14 Change in Words

FX-322-113: Hearing Signal and Speech Perception Improvements Observed in Subjects with Severe SNHL Double-blind, placebo-controlled study of 31 individuals randomized 4:1 • Pure tone average deficit between 71-90 decibel hearing level (dBHL) • Potential cochlear implant candidates Improvements in Bamford-Kowal-Bench Sentence-in-Noise exam (BKB- SIN) observed in treated ears • BKB-SIN measures signal-to-noise ratios required for subjects to correctly repeat words in sentences • Four FX-322 treated subjects show improvement, two with a 6 dB response • A single placebo subject showed a 3.6 dB change • No improvements observed in words-in-quiet Favorable safety profile • No treatment-related SAEs © Frequency Therapeutics, Inc. | 15 © Frequency Therapeutics, Inc. | 15
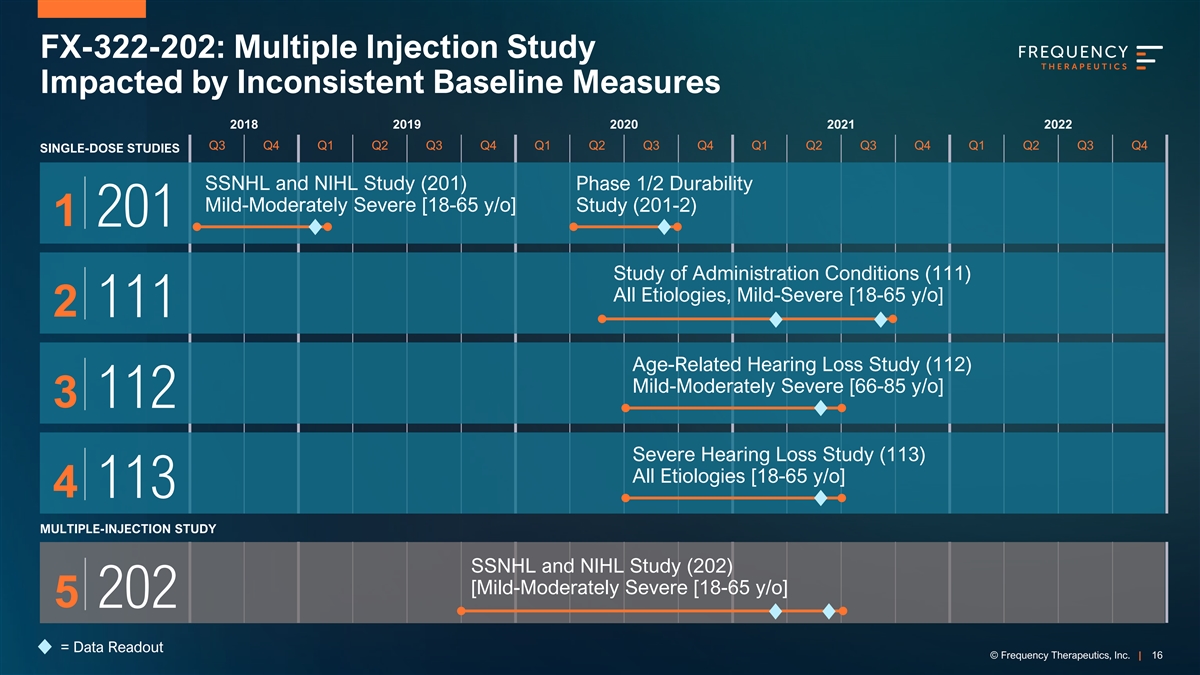
FX-322-202: Multiple Injection Study Impacted by Inconsistent Baseline Measures 2018 2019 2020 2021 2022 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 SINGLE-DOSE STUDIES SSNHL and NIHL Study (201) Phase 1/2 Durability Mild-Moderately Severe [18-65 y/o] Study (201-2) 1 201 Study of Administration Conditions (111) All Etiologies, Mild-Severe [18-65 y/o] 2 111 Age-Related Hearing Loss Study (112) Mild-Moderately Severe [66-85 y/o] 3 112 Severe Hearing Loss Study (113) All Etiologies [18-65 y/o] 4 113 MULTIPLE-INJECTION STUDY SSNHL and NIHL Study (202) [Mild-Moderately Severe [18-65 y/o] 5 202 = Data Readout © Frequency Therapeutics, Inc. | 16

Comparing Pooled Data to Multiple-Injection Study FX-322-202 Placebo-Treated and Untreated Ears are Outside 95% Confidence Interval % Exceeding % Exceeding Single-dose Studies Multiple Injection Study 95% Confidence 95% Confidence Interval Interval (201, 111, 112) (202) 25% 25% 21.2% 20% 20% 15.4% 14.1% 15% 15% 10% 10% 5% 5% 2.5% 2.5% 2.1% 0% 0% Untreated Untreated Literature* Treated Literature Treated and Placebo and Placebo n = 71 n = 66 n = 104 n = 97 95% confidence intervals established by Thornton & Raffin (1978) and modified by Carney & Schlauch (2007) © Frequency Therapeutics, Inc. | 17

Clinical Study Data Informs New FX-322 Phase 2b Study © Frequency Therapeutics, Inc. | 18

New Clinical Study FX-322-208 Designed to Advance Drug Candidate to Pivotal Trials Built upon insights Sufficient sample Reduce from trials with hearing size to demonstrate potential restoration signal efficacy for bias Etiology, severity, Multiple baseline Approach based baseline speech measures on pooled data perception Primary endpoint of Multiple speech speech perception perception tests © © F Fr req equ uen enc cy y T Th her era ap peu eut tiic cs s, I , In nc c. . | | 19 19

Pooled Single-Dose Studies (201, 111, 112) Data Suggest Patterns Between Etiology/Severity and Response Moderately- Moderate Mild Severe Severe 71 Treated with single-dose of FX-322 Noise The size of each circle represents the 0% 0% 33% (18-65 years) number of people tested per group The color of the circle represents the percentage of responders Sudden 208 Trial: Target Population 0% 27% 40% (18-65 years) 7-10 Million U.S. patients 208 Trial FX-322: Extended Population Age-related 0% 7% 0% (65+ years) 15+ Million U.S. patients © Frequency Therapeutics, Inc. | 20

Multiple Design Features Have Been Added to Mitigate Bias And Demonstrate Greater Separation Between Signal and Placebo Lead-in phase with multiple Start of 1-Month Lead-in baseline measures Day -30 Lead-in Sites and patients masked to Day -15 qualifying test results Baseline Day 1 All sessions recorded and monitored Ability to disqualify subjects based on symptom stability © Frequency Therapeutics, Inc. | 21

New FX-322 Placebo-Controlled Phase 2b Study Commenced First patient dosed in FX-322-208 Study in October 2021 Subjects Screened to Enter Lead-in Pure tone average 35-85dB 124 Subjects Start of 1-Month Lead-in Day -30 Subjects will have diagnosed Lead-in period with noise induced or sudden 3 visits determines Lead-in sensorineural hearing loss trial candidacy based Day -15 on consistency of audiologic testing Ages 18 – 65 Baseline Day 1 Randomize 124 subjects assumes 10% attrition Study powered at 80% FX-322 Placebo Effect size 20% over placebo Placebo 1X 1X Significance level is 0.05 1X N = 62 N = 62 N = 62 Follow-up Visits: Days 30, 60, 90 © Frequency Therapeutics, Inc. | 22

FDA Type C Meeting Held to Gain Alignment ALIGNMENT Primary Endpoint Gained alignment with FDA on speech perception as the primary endpoint 208 Study Design FDA reviewed and commented on 208 study, comments were incorporated into study protocol Patient Reported Outcomes (PRO) FDA feedback provided on novel PRO development called RADIAL; special meeting granted for further discussion © Frequency Therapeutics, Inc. | 23
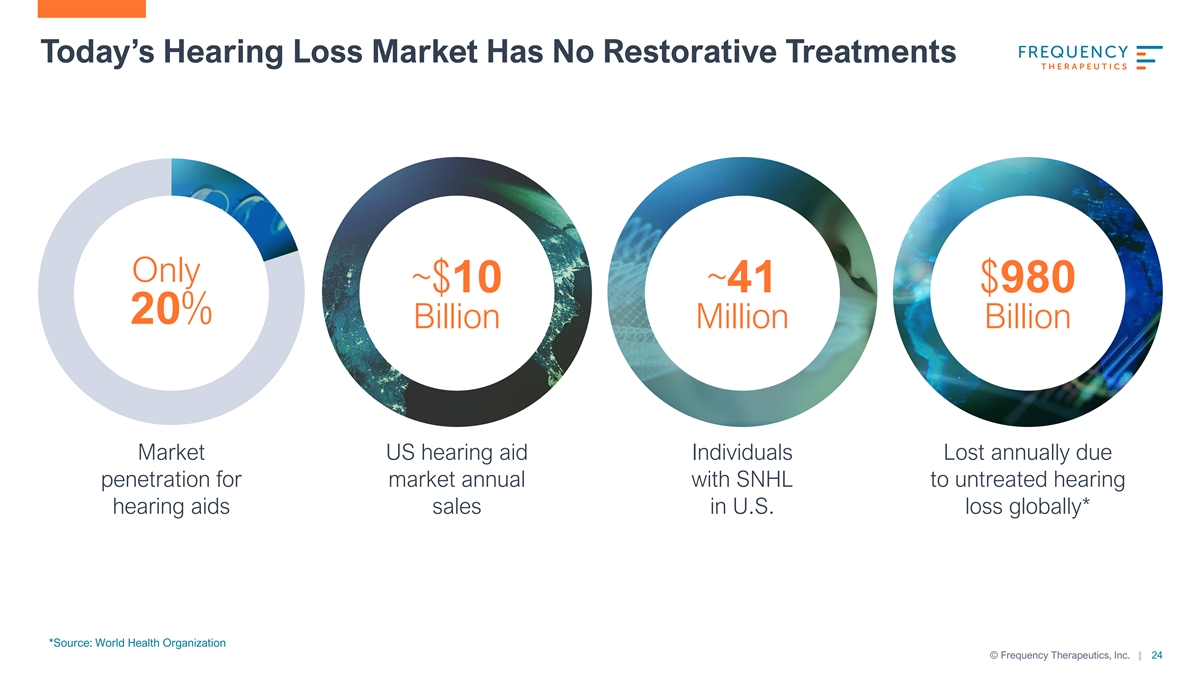
Today’s Hearing Loss Market Has No Restorative Treatments Only ~$10 ~41 $980 20% Billion Million Billion Market US hearing aid Individuals Lost annually due penetration for market annual with SNHL to untreated hearing hearing aids sales in U.S. loss globally* *Source: World Health Organization © Frequency Therapeutics, Inc. | 24

Hearing Loss Can Have a Significant Impact on Overall Health July, 2020 November, 2018 Increased risks with untreated hearing loss “Hearing loss is the largest potentially modifiable risk factor 50% 41% for developing dementia” Dementia Depression JAMA Nov 8, 2018, Deal J, et al. Incident Hearing Loss and Comorbidity. A Longitudinal Administrative Claims Study. © Frequency Therapeutics, Inc. | 25

Pipeline Expansion © Frequency Therapeutics, Inc. | 26

New Regenerative Program What if we were able to get drug deeper into the cochlea? © Frequency Therapeutics, Inc. | 27

FX-345 Working to Achieve Broad Exposure Through the Cochlea • Second clinical program focused on regrowth of sensory cells • Enables coverage of large portion of cochlea • Potential to address additional SNHL patient types • Formulation enabling evaluation of a range of dose levels • Developing in addition to FX-322. Clinical data will drive commercial positioning © Frequency Therapeutics, Inc. | 28

FX-345 – A New Development Candidate Creating Effective Drug Levels Through Large Portion of Cochlea © Frequency Therapeutics, Inc. | 29

FX-345 Path to Clinic Program start planned for H2:2022 for a Phase 1b study in patients with SNHL Enables us to clinically evaluate increased cochlear coverage across range of doses in multiple patient populations Candidate IND-enabling Phase 1b Phase 1b selection GLP tox Program Start Read Out On-going H2 2022 H1 2023 © © F Fr req equ uen enc cy y T Th her era ap peu eut tiic cs s, I , In nc c. . | | 30 30

New Regenerative Program What if we could extend our approach to other degenerative diseases? © Frequency Therapeutics, Inc. | 31

Novel Frequency Small Molecule Inhibitors Drive Oligodendrocyte Differentiation Lead Optimization generated FREQ-162 Highly potent Highly efficacious Orally bioavailable Brain penetrant Novel chemical entity Patent application filed Frequency discovered a novel and highly effective target 1 3 10 30 100 Developed novel chemical nM nM nM nM nM entities that are highly potent inducers of oligodendrocyte differentiation FREQ-162 © Frequency Therapeutics, Inc. | 32 % Newly Differentiated Oligodendrocytes Vehicle Negative Control

FREQ-162 Outperforms Literature Compounds In Vivo Adult mice received 3 doses of comparator compounds or a single dose of FREQ-162 Brains were stained for a marker of newly generated oligodendrocytes Single dose FREQ-162 induces more OPCs to differentiate than comparator compounds Vehicle T3 / Thyromimeticα-Lingo Antibody Clemastine / Anti-Muscarinic FREQ-162 x 3 days 10 mg/kg x 3 days 5 mg/kg x 3 days 75 mg/kg x 3 days 5 mg/kg, Single Dose FREQ-162 induces formation α-Lingo antibody: Clemastine: Thyroid Hormone: of newly differentiated Thyromimetic Class Blocking antibody Anti-Muscarinic Class oligodendrocytes in both white and gray matter © Frequency Therapeutics, Inc. | 33

The Cuprizone Model of Chronic Demyelination Healthy Control Cuprizone, Vehicle Cortex Corpus Callosum Adult mice were demyelinated via 17 months of cuprizone Striatum administration • Elderly mice with long term demyelination Myelin Basic Protein Myelin Basic Protein © Frequency Therapeutics, Inc. | 34

FREQ-162 Outperforms Published Compounds In Vivo Adult mice received up to 10 daily doses of comparators or a single dose of FREQ-162 Brains were stained for Myelin Basic Protein (green) Single dose FREQ-162 induces more remyelination than comparator compounds Vehicle T3 / Thyromimeticα-Lingo Antibody Clemastine / Anti-Muscarinic FREQ-162 x 10 doses 10 mg/kg x 10 doses 5 mg/kg x 3 doses 75 mg/kg x 10 doses 5 mg/kg, Single Dose FREQ-162 induces formation α-Lingo antibody: Clemastine: Thyroid Hormone: of new myelin in white and Lingo inhibitor Anti-Muscarinic Class Thyromimetic Class gray matter Animals demyelinated for 17 months via cuprizone treatment © Frequency Therapeutics, Inc. | 35

Frequency NCEs Outperform Competitors: High Magnification FREQ-162 Vehicle Cortex Cortex High Magnification view reveals that FREQ-162 Corpus Callosum Corpus Callosum yields myelination • in both white and gray matter • In the appropriate orientation and location Striatum Striatum 5 mg/kg x 1 dose Myelin Basic Protein © Frequency Therapeutics, Inc. | 36

FREQ-162: Highly Reproducible Increases in Myelination All 8 out of 8 mice treated with FREQ-162 showed robust increases in myelination in both white and gray matter tracts Vehicle #1 #2 #3 #4 #5 #7 #6 #8 FREQ-162 #1 #2 #3 #4 #5 #7 #6 #8 Myelin Basic Protein © Frequency Therapeutics, Inc. | 37

Freq-162 Induces Robust 6000 Increases in Myelination Myelin Basic Protein 5000 • Forebrain myelin basic protein levels 4000 quantitated • A single dose of a Frequency compound induces robust remyelination 3000 2000 Dose Fold Compound # of doses P= (mg/kg) change α-Lingo antibody 5 3 0.9 x 0.99 Clemastine 75 10 1.7 x 0.70 1000 Thyroid Hormone (T3) 10 10 1.4 x 0.95 FREQ-162 5 1 7.7 x <0.0001 0 Healthy Vehicle T3 Lingo Ab Clemastine FREQ-162 (10 mg/kg) (5 mg/kg) (75 mg/kg) (5 mg/kg) Naive © Frequency Therapeutics, Inc. | 38 MBP (Intensity Weighted Pixel Count)

Remyelination: Path Forward Discovered novel target Generated multiple compounds Induced high levels of oligodendrocyte differentiation and remyelination in vivo Initiating IND enabling studies © Frequency Therapeutics, Inc. | 39

Our Path Forward We believe FX-322 restores hearing. We know characteristics of FX-322 responders. Learnings from previous trials informed new trial design with strong controls and FDA aligned clinical endpoints. We have a compelling new hearing program that will allow us to explore the impact of going deeper into the cochlea. We also have an exciting remyelination program in multiple sclerosis with a novel target and a strong response in vivo. We are a well capitalized company with resources to deliver innovation for patients and value for investors. • $142.4m in cash and cash equivalents*, runway into 2024 • Ex-US partnership with Astellas, significant milestones and royalties *Number reflects unaudited Cash, Cash Equivalents, and Marketable Securities as of December 31, 2021, and does not include Restricted Cash © Frequency Therapeutics, Inc. | 40

Appendix

Broad Potential of Progenitor Cell Activation Approach 4 © Frequency Therapeutics, Inc. | 42 2

Origin of Frequency Therapeutics Tissue-Specific, Pre-programmed Stem Cells Decoding Enabling Frequency Intestinal Cochlear Therapeutics Regeneration Regeneration Small molecule Langer and Karp publish Same cues reactivate therapeutics show small molecules activate normally inactive clinical proof intestinal progenitors progenitors in the cochlea of concept Niche-independent high-purity cultures of Clonal Expansion of Lgr5-Positive Cells Lgr5+ intestinal stem cells and their progeny from Mammalian Cochlea and High- Purity Generation of Sensory Hair Cells © Frequency Therapeutics, Inc. | 43

Frequency Progenitor Cell Activation (PCA) Approach Inactive Progenitor Cell Inactive ACTIVATED Progenitor Progenitor Asymmetric division using native programs Functional Target Cell Combinations of small molecules designed to activate progenitor cells © Frequency Therapeutics, Inc. | 44

Uniqueness of Our PCA approach Previous Approaches Frequency’s PCA Approach Pluripotent Multipotent Yamanaka 4 Factors Partial Reprogramming Bipotent Transdifferentiation Fully Differentiated Hair Progenitor Hair Progenitor Cell Cell Cell Cell © Frequency Therapeutics, Inc. | 45

Our Approach: Activation of Progenitors to Replace Hair Cell Loss Despite Hair Cell Loss, Progenitor Cells Remain Audiogram Human Cochlea Cross-section 47 Year Old Male with Occupational Noise Deafness © Frequency Therapeutics, Inc. | 46

Profound Synergy Between Pathways to Regenerate Cells Culture Media Wnt Activation (glycogen synthase kinase-3 Cochlear Progenitor (GSK3) Inhibitor; NCE) Proliferation (Lgr5+ – GFP) HDAC Inhibition (sodium valproate) Wnt Activation + HDAC inhibition PROFOUND SYNERGY HDAC = Histone deacetylase NCE = new chemical entity In vitro mouse model testing © Frequency Therapeutics, Inc. | 47

FX-322 Agents Induce Protein Expression Consistent with Fully Functional Sensory Hair Cells Hair cells Hair cells Hair cells Hair bundles Transducing cell dye Synapses Sensing Sound Creating Signal Transmitting Signal Generating intricate Producing functional Synaptic proteins to communicate hair bundles ion channels with nerve are present McLean et al., 2017, Cell Reports 18, 1917–1929 February 21 © Frequency Therapeutics, Inc. | 48 http://dx.doi.org/10.1016/j.celrep.2017.01.066

Images Showing Cellular Regeneration In Vivo Hearing Loss Model VEHICLE CONTROL TREATED WITH FX-322 HAIR CELLS NUCLEI HAIR CELLS NUCLEI Representative of n=7; Numbers correspond to frequencies; 30 days after treating © Frequency Therapeutics, Inc. | 49

Clinically Meaningful: 10% Means Needing Audiologic Help Words 100% 50 May get by with 90% 45 consumer technology and lifestyle changes. Difficult communication, 80% 40 +10% especially in noise. Challenges to home 70% 35 and work relationships. 60% 30 Needs help. -10% Can no longer communicate in person or on phone 50% 25 without professional audiologic help. 40% 20 30% 15 20% 10 10% 5 0% 0 © Frequency Therapeutics, Inc. | 50

Clinically Meaningful: 10% Means Functional Deafness or Need for Implant Words 100% 50 90% 45 80% 40 70% 35 Can communicate using hearing aids and 60% 30 accommodations at home Unable to communicate, and work. 50% 25 +10% even with hearing aids. At risk for depression 40% 20 due to impact on home 30% 15 -10% and work. Cochlear implants 20% 10 or functionally deaf. 10% 5 0% 0 © Frequency Therapeutics, Inc. | 51

Astellas Collaboration: Ex-US Development and Commercialization of FX-322 • Development and commercialization collaboration for FX-322, including lifecycle improvements • Astellas has ex-US rights; Frequency retains US rights to FX-322 Strategic commitment to invest in • Payments of up to $625mm which included $80mm upfront ENT as a therapeutic area - Development milestone payments to Frequency of ————— $65.0 million and $25.0 million upon the first dosing of a Research focus in regenerative patient in a Phase 2b clinical trial for SNHL in Europe and medicine Asia, respectively ————— - $100.0 million and $40.0 million upon the first dosing of a Global footprint in major markets patient in a Phase 3 clinical trial for SNHL in Europe and and distributorship model in Asia, respectively Africa/ME and LATAM • Development & commercialization: Astellas responsible for execution and costs of ex-US clinical development and commercialization © Frequency Therapeutics, Inc. | 52

Proven Leadership Team David Lucchino Chris Loose, Ph.D. Carl Lebel, Ph.D. President, CEO Chief Scientific Officer Chief Development Officer & Co-Founder & Co-Founder Chief Scientific Officer of Otonomy (2009 to 2016). Former CEO of Entrega Bio Co-founder/CTO of Semprus Executive Director, Amgen. (PureTech). Co-founder / CEO of BioSciences through FDA / CE Scientific fellow of the American Semprus BioSciences (acquired), clearance and acquisition. Princeton, Academy of Otolaryngology. Polaris Partners. MIT Sloan Fellow. MIT, Hertz Fellow and Yale Faculty. Dana Hilt, M.D. Sue Stewart, J.D., LLM Wendy Arnold Chief Medical Officer Chief Regulatory Officer Chief People Officer Neurologist and neuroscientist CRO at numerous biopharma HR leader with extensive life science with two decades in biopharma companies including Kaleido experience including senior leadership and CNS drug development. Biosciences, Candel Therapeutics, roles at Kaleido Biosciences, Amgen, Lysosomal, Forum and regulatory leadership roles at Moderna, Celgene Avilomics Pharma. Tokai Pharma, Transmolar and Research, and Inotek Pharmaceuticals Genzyme Corp. Quentin McCubbin, Ph.D. Chief Manufacturing Officer Led pharmaceutical sciences and process chemistry at Takeda / Millennium and headed technical operations Cerevel Therapeutics. © Frequency Therapeutics, Inc. | 53

Scientific Advisory Board Clinical Advisory Board Jeff Karp, Robert Langer, Robin Franklin, Sheng Ding, Dan Lee, Rene Gifford, Steve Rauch, Ruth Litovsky, Ph.D. SC.D. Ph.D. Ph.D. M.D. Ph.D. M.D. Ph.D. Associate Professor at David H. Koch Institute Professor of Stem Senior Investigator, Director, Pediatric Associate Director of Director, Vestibular Professor, Brigham and Women’s Professor at the Cell Medicine, Gladstone Otology and Pediatric Audiology, Division, Medical Communications Sciences Hospital, Harvard Massachusetts Institute Wellcome Trust-MRC Institute of Neurotology, Mass Director of Cochlear Director, Mass. Eye and Disorders and Surgery Medical School of Technology Cambridge Cardiovascular Eye and Ear Implant Program, and Ear Balance and Division of Otolaryngology, Stem Cell Institute Disease Vanderbilt University Vestibular Center University of Wisconsin Sean J. Siddhartha Amy Wagers, Chris Runge, Joni Doherty, Julie Arenberg, David Friedland, Morrison, Ph.D. Mukherjee, Ph.D. Ph.D. MD, Ph.D. MS, Ph.D. M.D., Ph.D. M.D., D.Phil. Director of the Forst Family Professor Chief of the Division Assistant Professor Associate Director Vice-Chair of the Children's Medical of Stem Cell and of Communication of Clinical of Clinical Audiology Department of Assistant Professor Center Research Regenerative Biology, Sciences, Medical Otolaryngology-Head for Research and Otolaryngology and of Medicine, Institute, Harvard University College of Wisconsin and Neck Surgery, Education, Mass Eye Communications Sciences, Columbia University UT Southwestern Keck School of and Ear Medical College of Medical Center Medicine of USC. Wisconsin © Frequency Therapeutics, Inc. | 54

Pioneering a New Category in Regenerative Medicine Frequency Therapeutics Corporate Presentation April 2022
